Powered by purpose
a pipeline inspired by patients
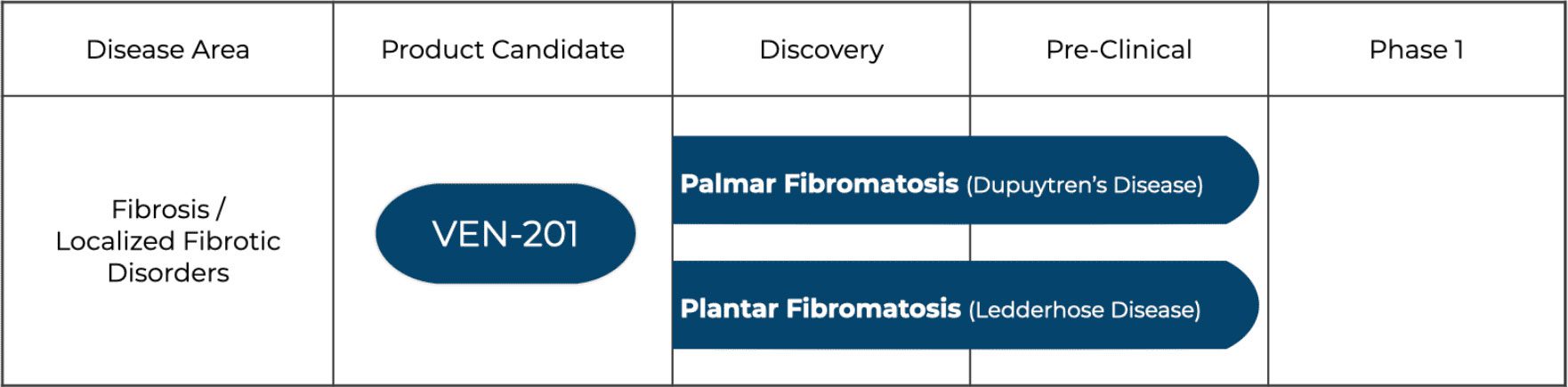
We are targeting under-served, localized fibrotic diseases that are chronic, progressive, and debilitating. Our focus with our lead investigational compound VEN-201 is on addressing the significant unmet medical need that remains with Dupuytren’s Disease and Ledderhose Disease.
Pipeline
VEN-201, an investigational compound, demonstrated key anti-fibrotic actions and a positive safety profile in a pre-clinical in vivo study using an established dermal fibrosis (scleroderma) model. Based on the demonstrated anti-fibrotic effects we are working to advance VEN-201 as our lead developmental candidate targeting Dupuytren’s and Ledderhose disease.
The investigational products listed on this page are not approved by the FDA or have not been approved for the above referenced indication, and the safety and effectiveness of such has not been established.

VEN-201 was initially identified via targeted scientific evaluation, expert opinion, and leveraging articifial intelligence data. Our goal was to identify previously approved treatments that have new potential as anti-fibrotic treatments for Dupuytren’s disease. The reference compound for VEN-201 has been previously approved by the FDA and EMEA for use in other indications for over 20 years, with a proven safety profile. Based upon our pre-clinical evaluation of VEN-201 as a potential anti-fibrotic agent, we are working to advance the compound as a repurposed, reformulated, disease modifying agent with a novel route of administration – via an expedited regulatory pathway.






